|
|
|
Home |
|
Back to Current Research |
|
Researchers |
|
Contact Us |
|
|
|
|
|
The Mechanism of SNARE-Mediated Fusion
|
|
|
| Cryo-EM of proteoliposomes |
|
|
 |
|
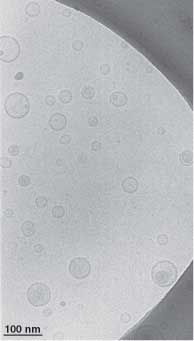
In 1998 we established the central function of the SNARE proteins as fusogens when we reconstituted these proteins into small unilamellar liposomes (shown on the left) and measured lipid transfer between liposomes containing cognate SNARE proteins. This system also lead to a nearly complete characterization of the yeast SNARE proteome. We have since developed a series of other reconstitution platforms, including supported bilayers which lead to the first sub-second measure of single SNARE fusion events and into Giant unilamellar liposomes (shown below, note the scale bar differences in the two figures) which are allowing a variety of surface diffusion measurements of SNAREs entering into various complexes. |
|
|
|
 |
|
|
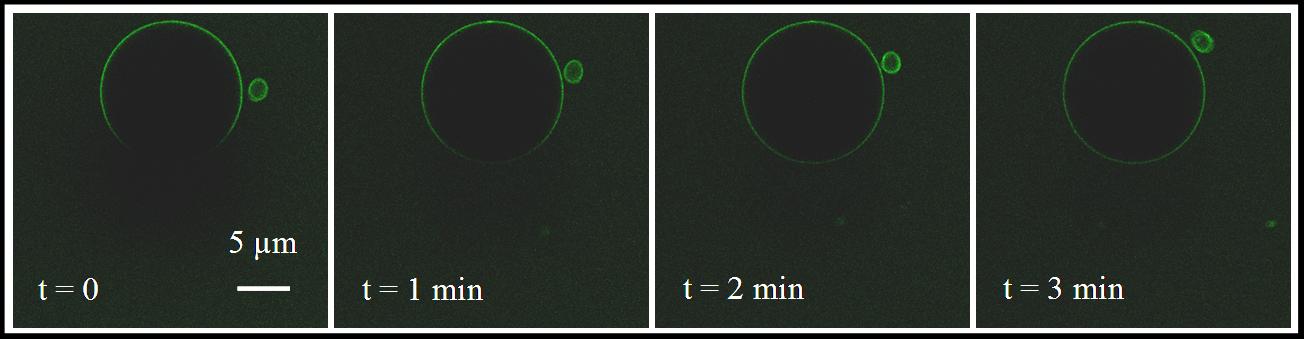
Giant v-SNARE proteoliposomes probed with fluorescently tagged soluble t-SNARE illustrates free mobility of SNARE complexes (FRAP). |
|
|
|
|
|
| Through the combination of these different platforms, we are measuring single fusion events by Total Internal Reflection Fluorescence Microscopy, we are determining SNARE-interaction energies from Vesicle Micromanipulation approaches and we are characterizing single fusion pores by electrophysiology. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|



